CDC Votes to Revise Hepatitis B Vaccine Guidelines for Newborns
The U.S. Centers for Disease Control and Prevention (CDC) has voted to change its long-standing recommendation that all newborns receive a hepatitis B vaccine at birth. This decision, made by the Advisory Committee on Immunization Practices (ACIP), may impact the preventative health landscape for infants in the U.S.
Why It Matters
This shift in vaccine policy raises significant questions about public health strategies aimed at protecting the most vulnerable populations, namely newborns. The new recommendations encourage discussions about vaccination based on a mother’s hepatitis B status, potentially leading to increased risks for unprotected infants. The implications of this decision could reverberate through American healthcare, particularly concerning the prevention of a virus that poses grave health risks.
Key Developments
- The ACIP voted on December 5 to change the hepatitis B vaccine guidelines, shifting to a risk-based approach rather than universal vaccination at birth.
- If mothers test negative for hepatitis B, the committee advises discussing the need for the vaccine with healthcare providers.
- Babies who do not receive the initial dose at birth should be vaccinated no earlier than two months of age.
- The committee’s decisions come after Health and Human Services Secretary Robert F. Kennedy, Jr., appointed new members, having dismissed the previous panel.
Full Report
Concerns About Exposure Risk
During the discussion leading to the vote, several committee members raised concerns about vaccinating children they deemed "low-risk" for hepatitis B infection. However, the asymptomatic nature of the virus complicates assessments of exposure risk. Hepatitis B can be transmitted via bodily fluids and can survive on surfaces for extended periods, creating hidden risks for newborns, even if their mothers test negative for the virus.
Protecting Newborns
Some ACIP members contended that the birth dose of the hepatitis B vaccine served primarily to protect higher-risk populations. However, experts refute this claim, emphasizing that newborns are particularly vulnerable to severe complications from hepatitis B. Approximately 90% of infants infected with the virus face a significant risk of developing chronic infections, which can ultimately lead to liver disease or cancer.
Efficacy of the Current Vaccine Strategy
Dr. H. Cody Meissner, a committee member opposed to the new recommendations, pointed out the success of the birth-dose vaccination strategy in reducing infection rates significantly. The CDC reports that the annual cases of hepatitis B infection have decreased from several hundred thousand to just around 14,000, largely due to successful vaccination programs.
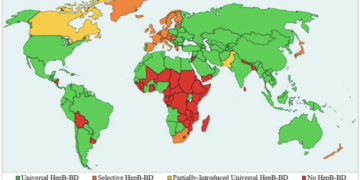
Global Vaccination Context
The committee’s decision reflects comparisons with other countries’ vaccination policies, including Denmark, which recommends hepatitis B vaccines only for infants whose mothers are infected. However, unlike the U.S., Denmark has a universal healthcare system that facilitates easier tracking of patient health records and prenatal care. As of September 2025, many countries globally continue to recommend universal hepatitis B vaccinations for newborns, reinforcing that the U.S. is not an outlier in this regard.
Context & Previous Events
The CDC has recommended that all infants receive hepatitis B vaccinations at birth since around 1991. This historical guidance aimed to curtail the spread of a virus that can lead to severe health issues later in life. Prior to the implementation of universal vaccination, rates of hepatitis B infections among children were significantly higher, with thousands of new cases reported annually. The change in policy announced this December represents a notable shift in the approach to pediatric vaccination in the United States.